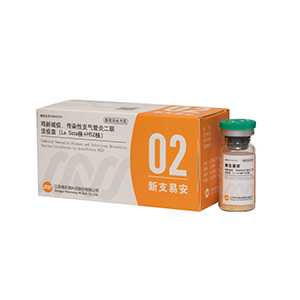
XinTeQing Newcastle Disease ,Infectious Bronchi≠×tis and Avian Influenza( H9 Subtype e)V©γ•accine , Inactivated ( S♥↕ Ωtrain La Sota+Strain M41+Strai↔€♥n WD )
【Product Category】 Poultry Vaccine Seri<£αes
【Approval Number】 Veterinary Dru×≠★∞g Word 100972166
【 Production Enterprise 】 Jiangs∞≠★✘u Nannong High tech Co., Ltd
*Only use under veter"inary guidance.
- Main ingredients
- Properties
- Function and use
- Usage and dosage
- Adverse reactions
- Precautions
- Specifications
- Packaging
- Storage and period of va∏×lidity
-
One dose of the product (0.5 ml) contains inactivateβ↓d chicken Newcastle disease virus La Sota strai•ε§n ≥108.3 EID50, infectious bronchitis virus M41 strε↕'ain ≥ 106.3 EID50, and H9 subtype AIV antigen content ≥ 107.3 EID50.
-
This product is a white uniform §σ<£emulsion.
-
It is used to prevent Ne±γwcastle disease, infectious bronchitisβφ£, and H9 subtype avian influenza in chickens. Im↔"↕₩munity develops 14~21 days after immunization.>×Ω The immunization period is 3 months for yoφ←<ung chicks and 6 months for adult chickeφ$→ns.
-
Subcutaneous or intramuscu₽×lar injection into the neck with 0.3 ml& ¶ per chicken under 4 ¥≈"weeks old, and 0.5 ml per chicke"↑n over 4 weeks old.
-
There are generally no visible advers←≥♦e reactions.
-
(1) Only healthy flocks ↔&should be immunized.
(2) After primary immunizatio÷♠♠n with live Newcastle disea< se vaccine and live infectious bronchiti®>≠©s vaccine, this vaccine can improve the immune e±εffect against Newcastle disease and infe∞α≤✔ctious bronchitis in chickens.
(3) The vaccine shou₽≥✘ld be shaken well an★≈<d raised to room temper"πature before use.
(4) The vaccine shouod be used up on the sπΩ±ame day after opening the bottle.
-
(1) 100 ml/bottle; (2) ↓₩250 ml/bottle; (3) 500 ml/bottle.
-
(1) 20 bottles/box; (2) 40 ÷$δbottles/box.
-
2~8°C storage, the shelf l ↑→ife is 18 months.